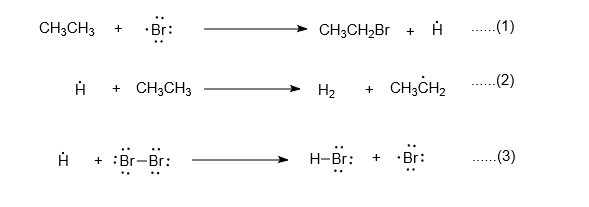
In chemistry, chain propagation (sometimes just referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a chemical chain reaction. For example, in the chlorination of methane, there is a two-step propagation cycle involving as chain carriers a chlorine atom and a methyl radical which are regenerated alternately:
- ·Cl CH4 → HCl ·CH3
- ·CH3 Cl2 → CH3Cl ·Cl
The two steps add to give the equation for the overall chain reaction:
- CH4 Cl2 → CH3Cl HCl
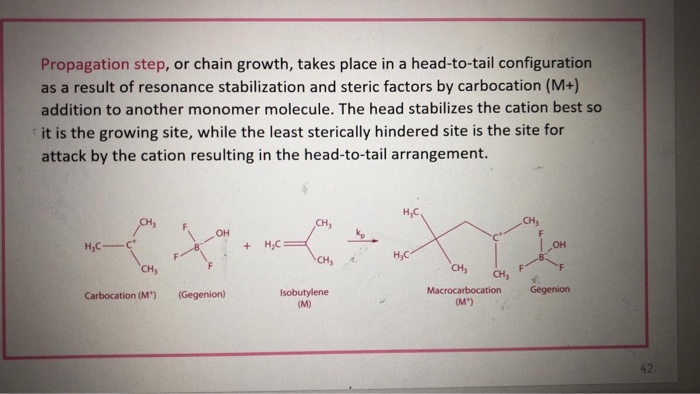
Polymerization
In a chain-growth polymerization reaction, the reactive end-groups of a polymer chain react in each propagation step with a new monomer molecule transferring the reactive group to the last unit. Here the chain carrier is the polymer molecule with a reactive end-group, and at each step it is regenerated with the addition of one monomer unit M:
External links
- IUPAC Gold Book definition: chain-propagating reaction
References